Effect

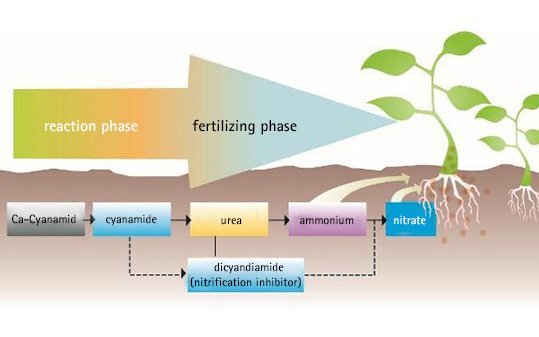
The decomposition of calcium cyanamide in soil:
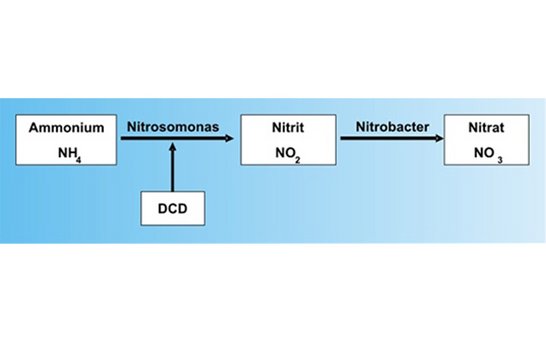
A part of the cyanamide reacts further into dicyandiamide (DCD). This DCD has nitrification inhibitory properties, i.e. it delays the conversion of the ammonium nitrogen into nitrate that is caused by certain soil bacteria (Nitrosomonas and Nitrobacter).
Schema of nitrification:
Dicyandiamide inhibits soley the activity of the Nitrosomonas bacteria, and not that of Nitrobacter. This means that no plant-damaging nitrite can accumulate in the soil. Generally speaking, calcium cyanamide converts in the soil via two phases:
Reaction phase and fertilizing phase
During the reaction phase calcium cyanamide undergoes the first steps of decomposition and develops a strong alkalinity. This creates a favourable environment for beneficial soil microbes and for a healthy growth of the crop. This phase continues for between 8 to 14 days in the soil depending on reaction conditions. The reaction rate depends on the moisture, temperature and activity of the soil, its humus content and the quantity of fertilizer applied.
It should be emphasized that no harmful residues remain in the soil. The cyanamide is completely transformed into urea, thereby improving the nitrogen nutrition of the plants. The nitrification inhibitor dicyandiamide also slowly decomposes into urea. This means that in the end everything is made available as a nutrient to the plants in the form of nitrogen and lime, with no non-soil decomposition products formed.
Nitrogen efficiency
Calcium cyanamide – providing an even and sustained supply of nitrogen to your plants
The nitrogen bound in the calcium cyanamide is not directly available to the plants.
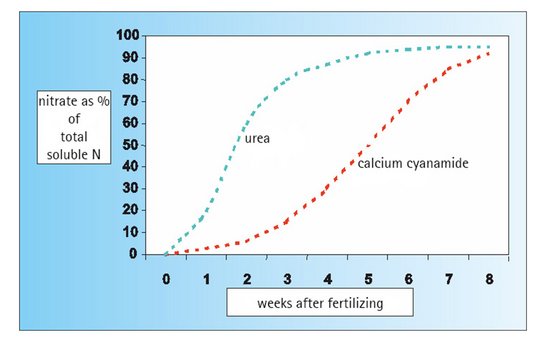
Ammonium (NH4), which can be taken up by plants directly through the roots, is only produced when the fertilizer is converted in the soil. With calcium cyanamide the further conversion of ammonium into nitrate nitrogen (=nitrification) is delayed (= inhibition of nitrification). This means that fertilizing with calcium cyanamide ensures that the crop is supplied with a nitrogen nutrient that has a strong ammonium emphasis and which is physiologically beneficial to the plants.
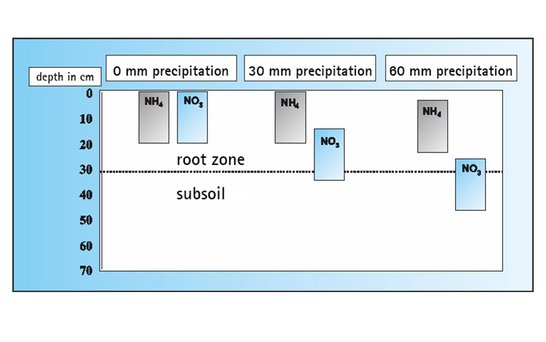
Unlike nitrate, which is easily movable in the soil solution, ammonium is bound to soil colloids (clay minerals). Therefore ammonium is not washed out, even during heavy precipitation, and can be absorbed by the plants gradually.
The gradual release of ammonium nitrogen from calcium cyanamide and the inhibition of nitrification cause the nitrogen to be evenly supplied to the plants over a long period of time. The result is a harmonious and healthy growth of the crop.
Lime effect
Calcium cyanamide – improves the structure of your soil
Lime is necessary:
- to maintain an optimum pH value
- to promote microorganisms
- to positively influence topsoil stability
- to control decomposition processes and humus formation
- to provide the plants with nutrients
Commercially available calcium cyanamide contains about 50% lime, with a third or a half respectively being present as free lime. The rest is chemically bound in calcium cyanamide and is released during conversion in the soil. This lime release has a particularly intensive effect because it is extremely reactive while in a nascent state.
Lime value:
The so-called lime value specifies the effect of a nitrogen fertilizer on the lime balance of a soil. If a fertilizer provides more lime than is required to neutralize the acids that are produced when nitrogen is converted in the soil, its lime value is positive. In the opposite case its lime value is negative, which means it consumes the lime content of the soil, reducing the soil's pH value.
Lime values of various nitrogen fertilizers
| Fertilizer | Lime gain or loss in kg CaO | |
|---|---|---|
| per 100 kg fertilizer | per 100 kg N | |
| PERLKA calcium cyanamide 19.8% N | + 30 | + 152 |
| CAN calcium ammonium nitrate, 27% N | - 16 | - 58 |
| Urea, 46% N | - 46 | - 100 |
| NPK, e.g. 13-13-21 | - 13 | - 100 |
| Ammonium sulphate nitrate | - 51 | - 196 |
| DAP 18 - 46 | - 37 | - 205 |
| Ammonium sulphate 21% | - 63 | - 300 |
As shown in the table below, an additional 152 kg of lime (calculated as CaO) is available per 100 kg N of PERLKA®. No other nitrogen fertilizert contains so much fast-acting calcium as PERLKA®.
Plant health
Calcium cyanamide ensures healthy growth
Calcium cyanamide promotes healthy growth in various ways:
- Plant growth and the resilience of the plants (firmer cell tissue) are positively affected as a result of the even, ammonium-accentuated nitrogen effect and the improved calcium supply.
- Calcium cyanamide increases the biological activity of the soil
- Rotting of the crop residues is accelerated
Soil fertility
Calcium cyanamide – so that your soil remains fertile
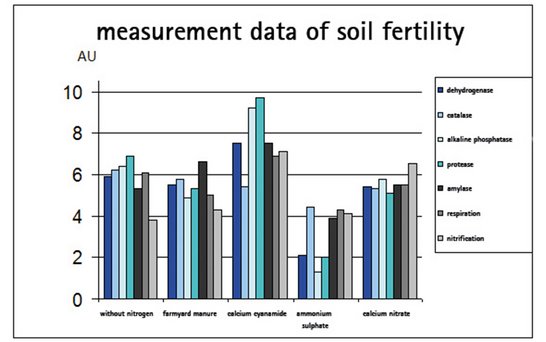
A long-term study carried out over 53 years by the Institute of Plant Nutrition at the Technical University Munich-Weihenstephan showed very clearly that using calcium cyanamide regularly resulted in a considerable increase in enzyme activity in the soil. This is regarded as a measure of the biological activity of the soil. Soil fertility is therefore significantly increased by the regular use of calcium cyanamide.
As the following graph shows, virtually all activity parameters are at their maximum in soils fertilized with calcium cyanamide.
Rotting acceleration
Calcium cyanamide for better rotting
The accelerated rotting of organic matter using calcium cyanamide was formerly used to produce so-called "artificial manure" or "propagating manure". Today calcium cyanamide is used in the manufacture of compost.
The resulting organic materials often have a wide C/N ratio of about 80:1. However, the microorganisms responsible for the rotting require a narrower C/N ratio of about 20:1. So nitrogen has to be added to the rotting material so that the bacterial flora can develop better. During the rotting process organic acids are generated as intermediate products. These need to be neutralized before they can be used to build valuable humic substances.
Calcium cyanamide fulfills these two conditions that are required for rapid rotting of organic substances - additional nitrogen and neutralization of acids.
Tests at the University of Goettingen have shown that unlike other fertilizers calcium cyanamide not only ensures accelerated rotting but also fully converts the organic material.
The accelerated rotting causes the compost heap to sag faster and stronger, thus ensuring a better use of space.
Adding calcium cyanamide increases the bacterial activity and enables the compost heap to reach the ideal temperatures.